For Academic Medical Centers and Cancer Centers
OnCore CTMS, along with integrated eReg, eConsent, and eSource + EDC platforms, is used by more AMCs and cancer centers than any other solution. For high-volume sites needing comprehensive operations and financial management, analytics, regulatory management, and data management, Advarra technology is the trusted leader.
For Health Systems, Hospitals, Sites, and Site Networks
Clinical Conductor CTMS is used by hundreds of research sites around the world to optimize research operations, manage financial workflows, boost patient recruitment, and much more. Integrated with world-class eRegulatory, eConsent, and eSource solutions, Clinical Conductor is built to streamline your research grow with your business.
TRUSTED BY CLINICAL RESEARCH LEADERS




A Better Trial Experience For Sites, Patients,
and Study Teams. And Better Results.
Principal Investigators (PIs) supported by Advarra technology
Research sites using Advarra solutions
of top NIH-funded institutions use an Advarra CTMS
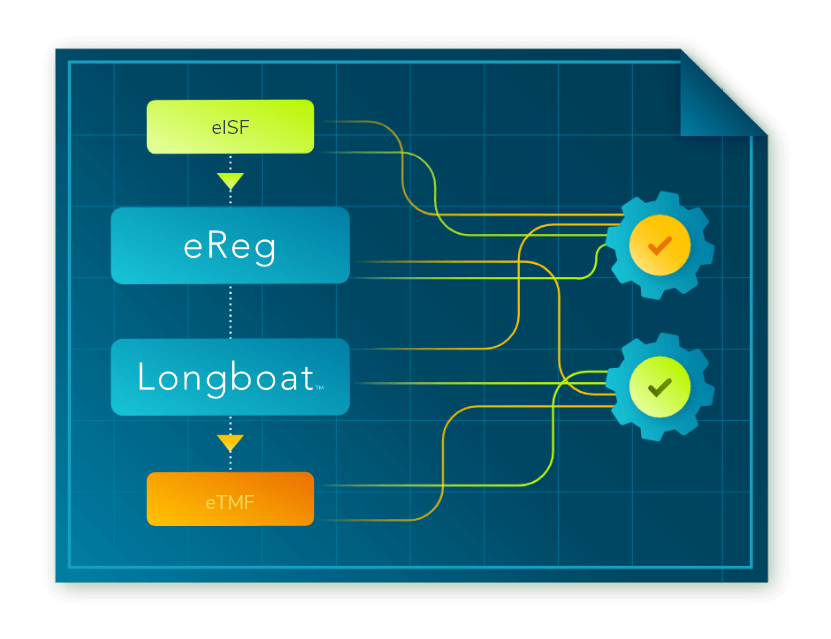
Secure Document Exchange: Connecting the Research Ecosystem
Secure Document Exchange allows all regulatory documents, originating with a sponsor or at a site, to be managed, signed, and securely exchanged seamlessly within the investigator site file (ISF) (eReg) or sponsor electronic trial master file (eTMF). Read about our approach and how industry-wide collaboration is the key to finally solving this longstanding challenge.
The Advarra Technology Solutions Experience
At Advarra, we have a special relationship with our customers because they are at the center of everything we do.
Our customers have personalized conversations on challenges they are facing every day and work with our teams to collaboratively develop solutions to those challenges. After working with the top cancer centers, academic medical centers, and health systems for more than two decades, we know what works, and can help you break the silos to accelerate clinical research.
Our customers include NCI-designated cancer centers, Clinical and Translational Science Award (CTSA) recipients, leading health systems, sites, site networks, and more.

We welcome all of our Technology Solutions customers to join the premier research community, Onsemble, a robust network of the nation’s leading clinical research professionals sharing best practices, addressing current challenges and supporting one another. We believe that by working together, we can reimagine the clinical trial experience and move research forward.
The Onsemble Community meets in person at our annual clinical research conference for several days of education, case studies, insights, and key takeaways for their institution to improve their research operations.

Benefits to Joining Advarra’s Onsemble Community
Help shape the software
you use every day
Collaboration is a core value at Advarra, and every major development project is completed in close collaboration with the organizations that benefit from our software.
Stay connected
with your peers
Our Onsemble Community Forum provides a connection to those in similar roles at peer institutions. With topics such as regulatory, training, CTO administration, and more, you will always be connected to an expert ready to help.
Find helpful resources,
just a click away
Join collaborations and group discussions, share useful files such as scripts, documentation, training materials, and more. You also gain access to our archive of Advarra-provided community resources.
Securing Your Data with a Next-Generation Cloud Platform
We are committed to the safety and security of your data, delivering technology solutions in an easy-to-use, fully managed cloud environment. We have received ISO 27001:2013 and SOC 2 Type 2 certification for our technology solutions, ensuring our products, services, and processes meet stringent requirements to enhance security and compliance.


